The continuing desire to reduce needlestick injuries led us to develop the EZ-fill® Integrated Safety System. This groundbreaking, fully passive needle shield comes ready to fill and requires no additional assembly steps.
ISS is both easy to use and intuitive to handle. Activating the automatic shield retraction requires less force compared to existing systems. As a fully passive system, it also prevents the end-user from any risk of piercing after the removal of the rubber needle shield, making it safe and easy to use.
ISS is delivered sterile and ready for filling, enabling pharmaceutical companies to add needlestick protection to their syringe products easily and quickly.
EZ-fill® ISS can increase the competitiveness of pharmaceutical products by reducing the complexity of production processes and logistics compared with other solutions. With no additional manufacturing steps, pharma companies do not have to invest in additional equipment, personnel or utilities.
ISS is validated in the 0.5mL* EZ-fill® syringe ½” needle with relative shelf-life.
*1mL under development

Reduction of logistics space
(incoming and outcoming)

Lower impact on secondary packaging
(blister and carton box)

No investment
for additional assembly machines

Minimal disruption
of existing prefilled syringe production and filling infrastructure

The design of the safety system
avoids unintentional activation of the mechanism during, filling, packaging and transport.

The elastomer tip cap
is also compatible with all major suppliers.

Anti-coagulant

Biologics

The shield, hub, and ring are molded using the latest plastic mold technologies in our ISO 13485-certified and FDA-inspected facility in Germany
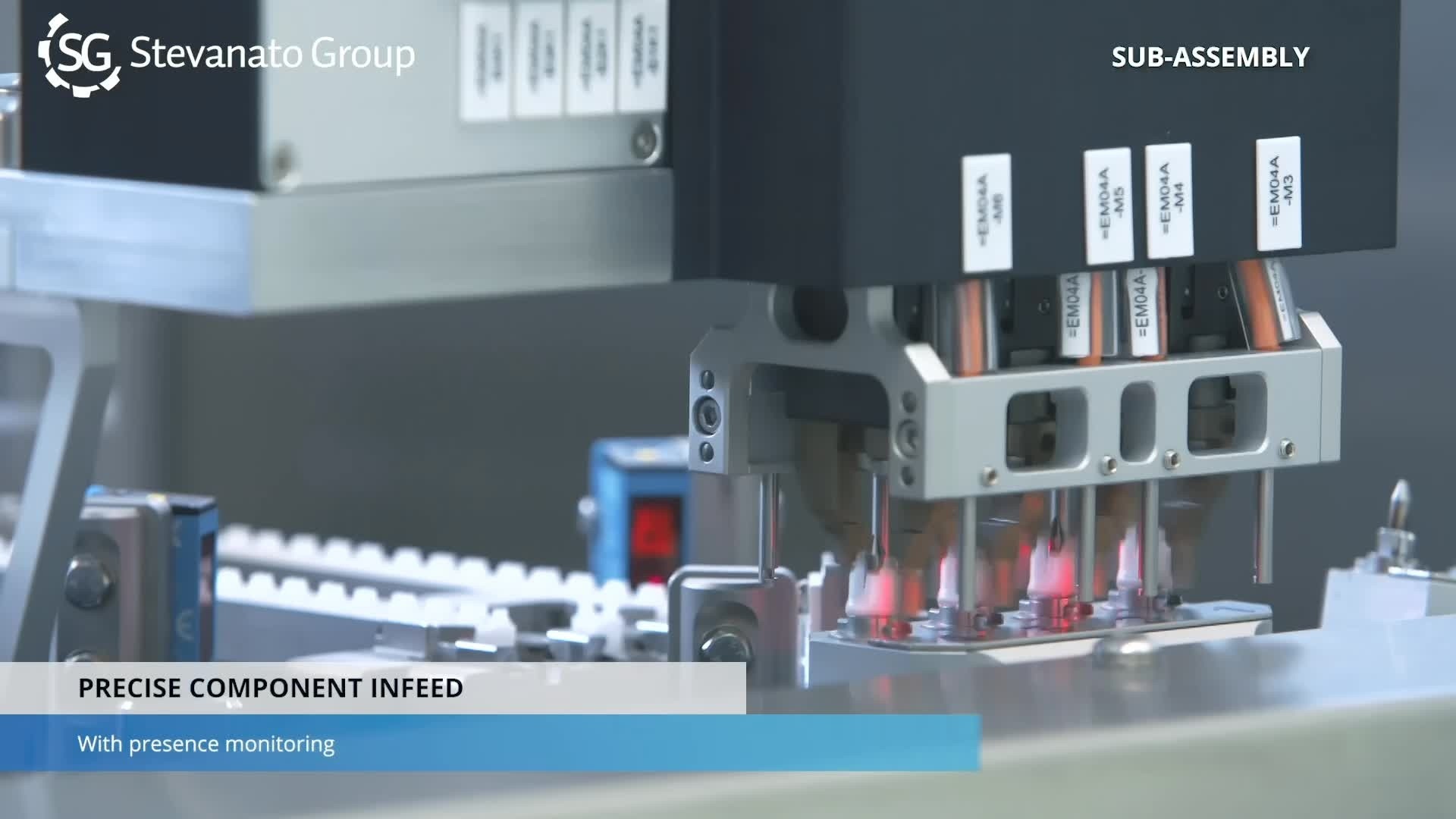
Sub-assembly of plastic components takes place in an ISO 5 cleanroom environment using assembly equipment. Each phase includes automatic inspection with high-accuracy sensors and cameras

The final assembly process is carried out on the EZ-fill® production line where the syringes are washed with WFI, siliconized and inserted into Nest & Tubs

ISS can be automatically inspected using our state-of-the-art vision inspection equipment, featuring seven camera controls and high-speed handling for full product integrity.